Can Chlorine and Bromine Form an Ionic Compound
Potassium and oxygen d. Can sulfur and bromine form an ionic.
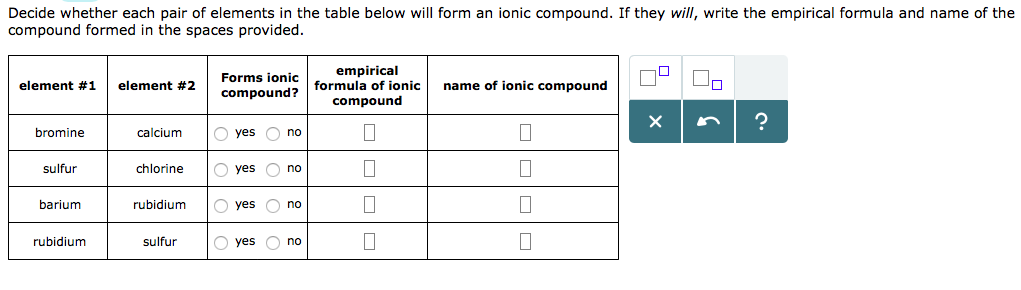
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
Lithium and chlorine b.
. The only truly non-polar bonds are between identical elements or compounds made with identical elements identically. I am taking Chem. E fluorine and sodium.
A Ionic compounds are formed by the transfer of electrons between the metals on the left side and the nonmetals on the right side. The charge on both the cation and. Up to 25 cash back Hello Both Chlorine and Bromine have 7 electrons in their outer shell and form ions by gaining one electronSo Chlorine becomes Cl- and Bromine.
Answer 1 of 2. Which of the following pairs of elements are likely to form an ionic compound. Which other element could most likely react to form a compound with.
View the full answer. Which of the following pairs of. Ionic compounds when two elements with a large electronegativity bond.
Oxygen and bromine c. Nitrogen hydrogern 011 Wht. What is compound of bromine and calcium.
Best Answer Copy Chlorine doesnt make an ionic bond with bromine because they both either need 1 electron or they both only take one electron. In thissodium is a metal having 1 oxidation state and Fluorine is a non-metal having -1 oxidation state which results in the formation of an ionic. This is the best answer based on feedback and ratings.
So the property of a bond determines more on the electronegativity difference than the EN of a single elements. Which pair of elements can form an ionic compound with each other. Lithium is a metal on the left side of the.
Step 1 of 3. CThe compound formed between rubidium and bromine is an ionic compound because rubidium is a metal and bromine is a non-metal. Up to 256 cash back Show transcribed image text A mixture of the compounds potassium bromide and potassium oxide contains 479 g of potassium bromide and is 585.
Chlorine can form ionic compounds like NaCl Sodium Chloride or CaCl2 Calcium Chloride but is not itself an ionic compound. The I ionic compound is. The elemental pairs that form ionic compounds are Sodium and Oxygen Manganese and Chlorine Oxygen and Calcium and Chlorine and Sodium.
Fluorine F chlorine Cl and bromine Br can all react easily to form compounds with sodium Na. Bromine is usually present in compounds. This is due to the weakness of.
Sodium and neon e. This problem has been solved. When calcium and bromine combine they form the ionic compound.
Although it has a greater melting and boiling point than fluorine and chlorine it has a lower melting and boiling point. To make an ionic bond. Ionic bonds are the.
IBr is best described as having polar covalent bonds.
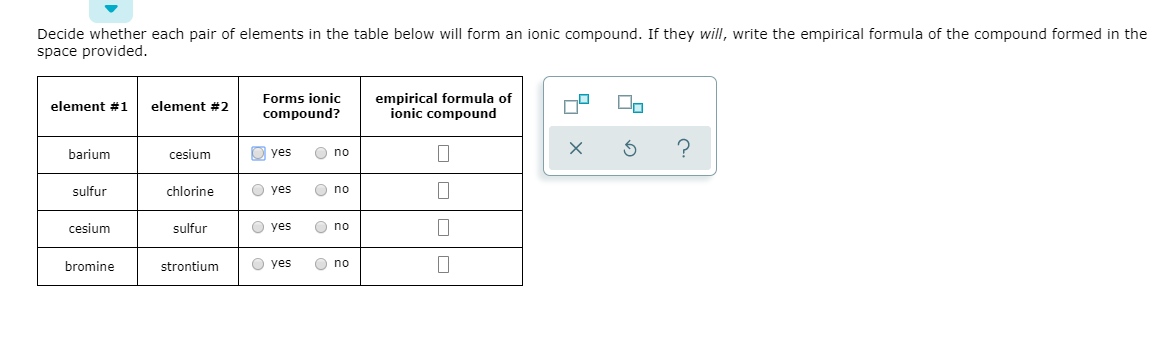
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
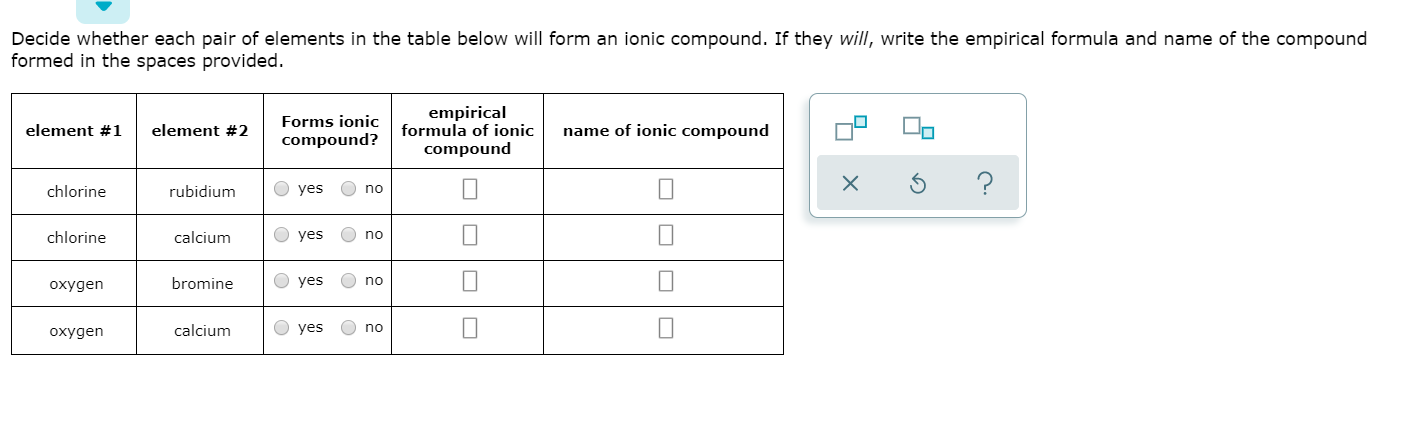
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com

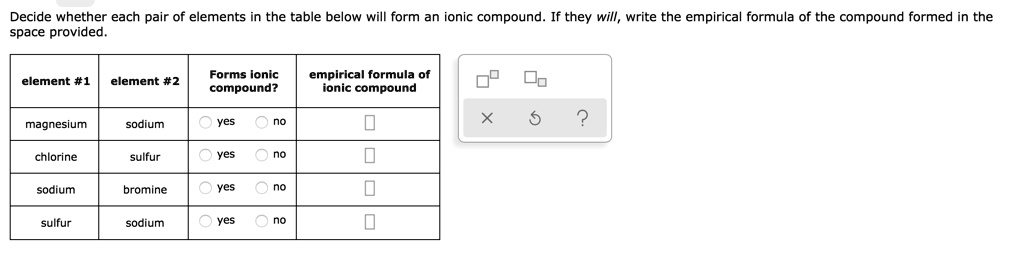
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula Of The Compound Formed In The Space Provided Forms Ionic
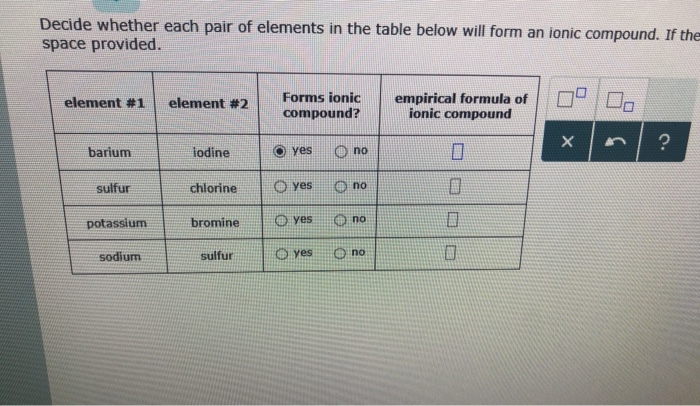
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
Comments
Post a Comment